After installation, run xhmm (with appropriate options): ./xhmm -p params.txtwhere an example of params.txt is given below.
NOTE:
It is assumed that the BAM files of "analysis-ready reads" being used have been generated using
the "best practice" bwa + Picard + GATK pipeline, though variations on this pipeline,
and even other pipelines to produce the BAM files for XHMM input, have been
successfully employed by various users.
For running GATK's DepthOfCoverage in parallel, first split up your samples' list of BAMs
into the desired degree of parallelism (in the example here, three files):
group1.READS.bam.list
group2.READS.bam.list
group3.READS.bam.list
Run GATK for depth of coverage (three times: once for samples in group1, once for group2, and once for group3):
java -Xmx3072m -jar ./Sting/dist/GenomeAnalysisTK.jar \
-T DepthOfCoverage -I group1.READS.bam.list -L EXOME.interval_list \
-R ./human_g1k_v37.fasta \
-dt BY_SAMPLE -dcov 5000 -l INFO --omitDepthOutputAtEachBase --omitLocusTable \
--minBaseQuality 0 --minMappingQuality 20 --start 1 --stop 5000 --nBins 200 \
--includeRefNSites \
--countType COUNT_FRAGMENTS \
-o group1.DATA
java -Xmx3072m -jar ./Sting/dist/GenomeAnalysisTK.jar \
-T DepthOfCoverage -I group2.READS.bam.list -L EXOME.interval_list \
-R ./human_g1k_v37.fasta \
-dt BY_SAMPLE -dcov 5000 -l INFO --omitDepthOutputAtEachBase --omitLocusTable \
--minBaseQuality 0 --minMappingQuality 20 --start 1 --stop 5000 --nBins 200 \
--includeRefNSites \
--countType COUNT_FRAGMENTS \
-o group2.DATA
java -Xmx3072m -jar ./Sting/dist/GenomeAnalysisTK.jar \
-T DepthOfCoverage -I group3.READS.bam.list -L EXOME.interval_list \
-R ./human_g1k_v37.fasta \
-dt BY_SAMPLE -dcov 5000 -l INFO --omitDepthOutputAtEachBase --omitLocusTable \
--minBaseQuality 0 --minMappingQuality 20 --start 1 --stop 5000 --nBins 200 \
--includeRefNSites \
--countType COUNT_FRAGMENTS \
-o group3.DATA
Combines GATK Depth-of-Coverage outputs for multiple samples (at same loci):
./xhmm --mergeGATKdepths -o ./DATA.RD.txt \
--GATKdepths group1.DATA.sample_interval_summary \
--GATKdepths group2.DATA.sample_interval_summary \
--GATKdepths group3.DATA.sample_interval_summary
Optionally, run GATK to calculate the per-target GC content and create a list of the targets with extreme GC content:
java -Xmx3072m -jar ./Sting/dist/GenomeAnalysisTK.jar \
-T GCContentByInterval -L EXOME.interval_list \
-R ./human_g1k_v37.fasta \
-o ./DATA.locus_GC.txt
cat ./DATA.locus_GC.txt | awk '{if ($2 < 0.1 || $2 > 0.9) print $1}' \
> ./extreme_gc_targets.txt
Optionally, run PLINK/Seq to calculate the fraction of repeat-masked bases in each target and create a list of those to filter out:
./sources/scripts/interval_list_to_pseq_reg EXOME.interval_list > ./EXOME.targets.reg
pseq . loc-load --locdb ./EXOME.targets.LOCDB --file ./EXOME.targets.reg --group targets --out ./EXOME.targets.LOCDB.loc-load
pseq . loc-stats --locdb ./EXOME.targets.LOCDB --group targets --seqdb ./seqdb | \
awk '{if (NR > 1) print $_}' | sort -k1 -g | awk '{print $10}' | paste ./EXOME.interval_list - | \
awk '{print $1"\t"$2}' \
> ./DATA.locus_complexity.txt
cat ./DATA.locus_complexity.txt | awk '{if ($2 > 0.25) print $1}' \
> ./low_complexity_targets.txt
Filters samples and targets and then mean-centers the targets:
./xhmm --matrix -r ./DATA.RD.txt --centerData --centerType target \
-o ./DATA.filtered_centered.RD.txt \
--outputExcludedTargets ./DATA.filtered_centered.RD.txt.filtered_targets.txt \
--outputExcludedSamples ./DATA.filtered_centered.RD.txt.filtered_samples.txt \
--excludeTargets ./extreme_gc_targets.txt --excludeTargets ./low_complexity_targets.txt \
--minTargetSize 10 --maxTargetSize 10000 \
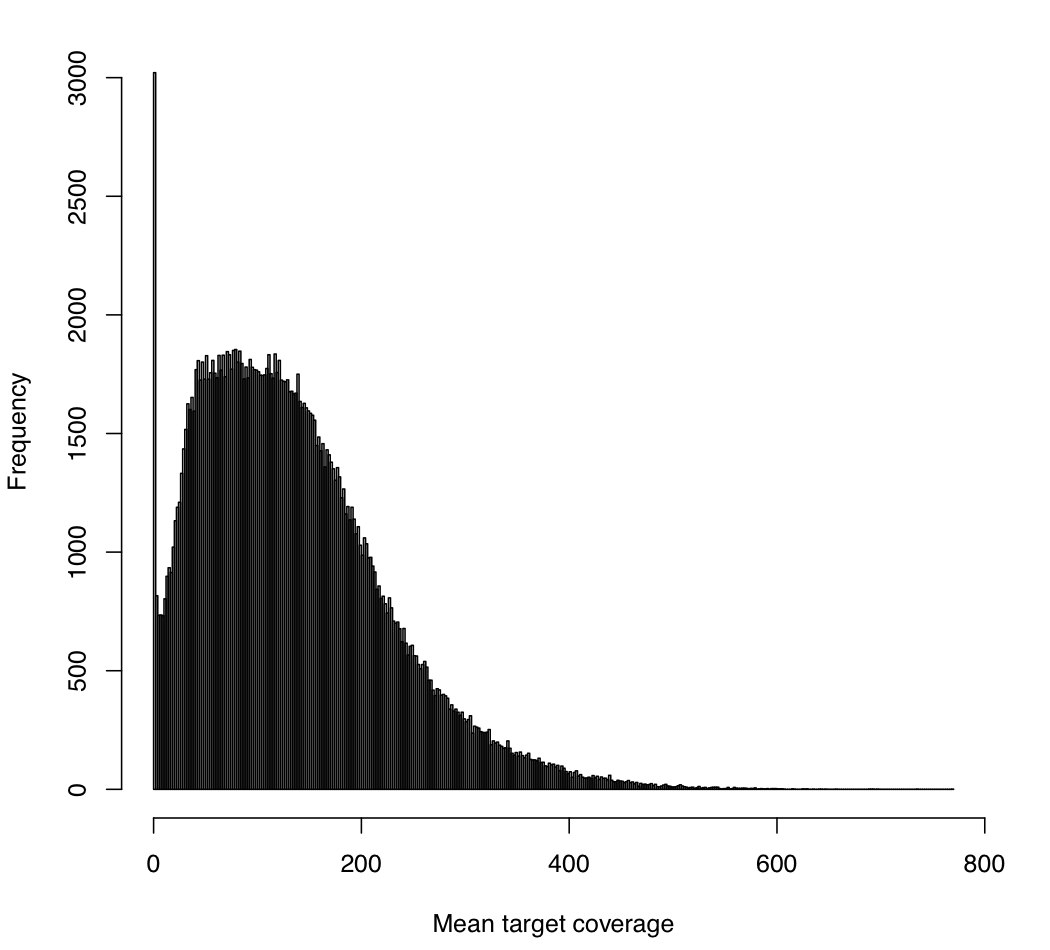
--minMeanTargetRD 10 --maxMeanTargetRD 500 \
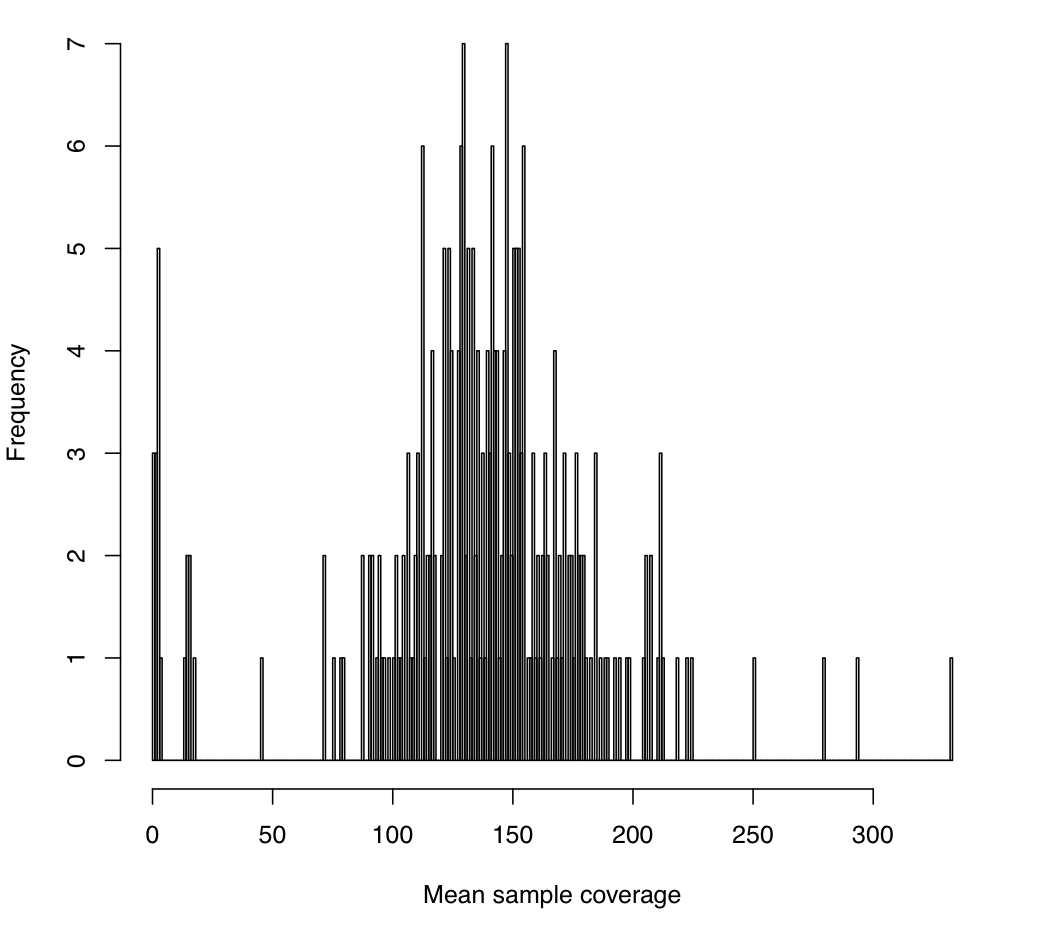
--minMeanSampleRD 25 --maxMeanSampleRD 200 \
--maxSdSampleRD 150
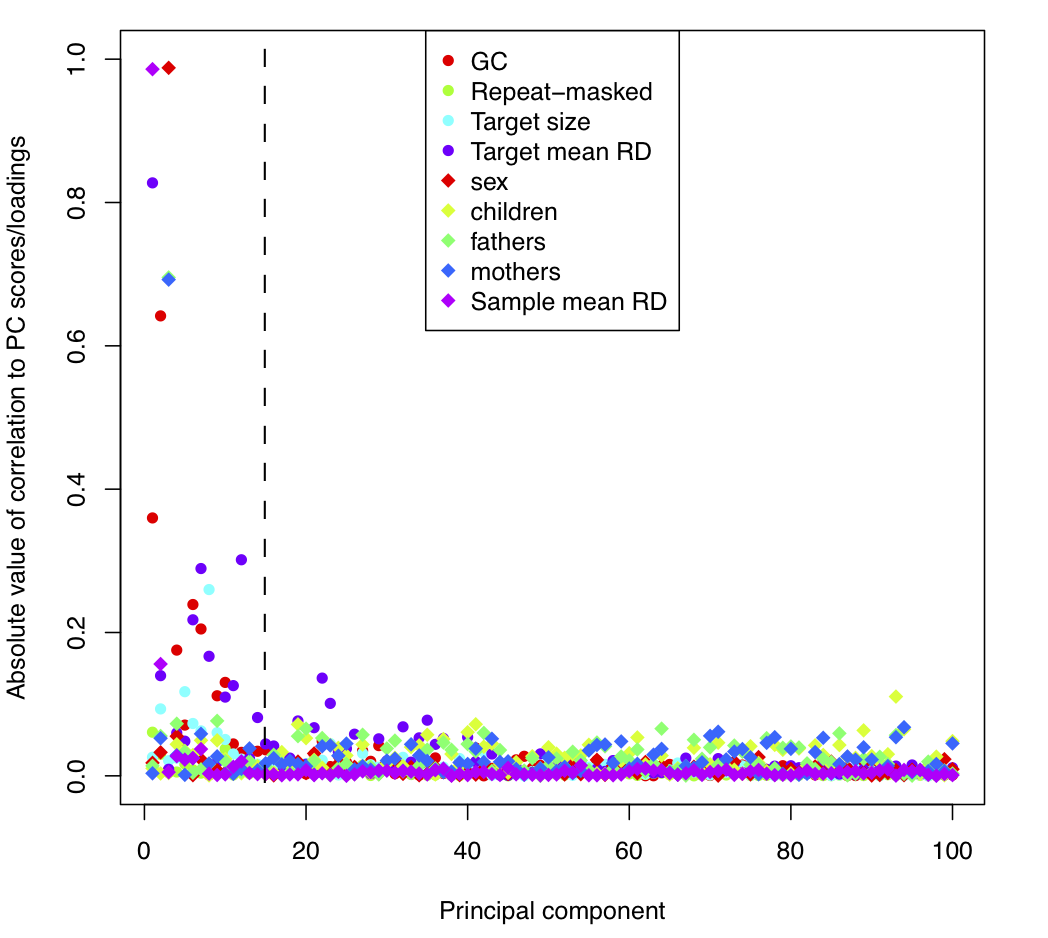
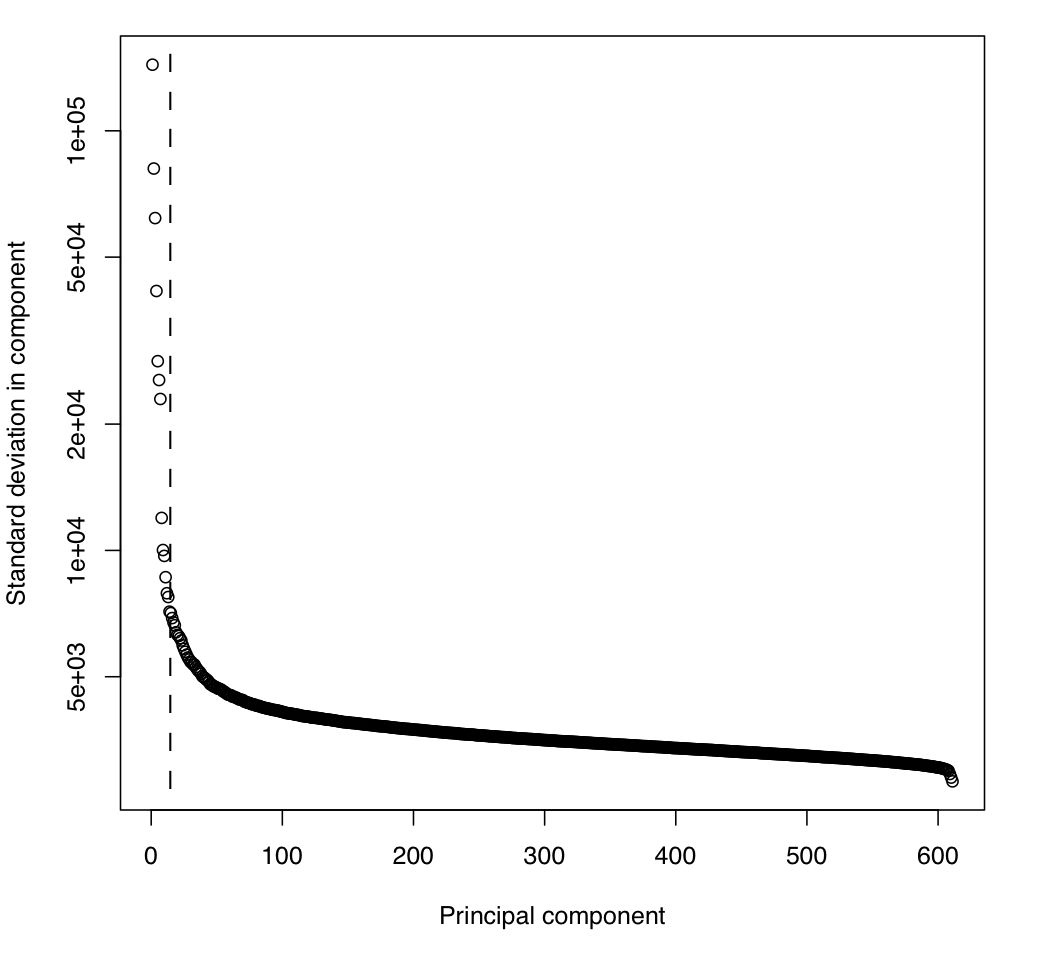
Runs PCA on mean-centered data:
./xhmm --PCA -r ./DATA.filtered_centered.RD.txt --PCAfiles ./DATA.RD_PCA
Normalizes mean-centered data using PCA information:
./xhmm --normalize -r ./DATA.filtered_centered.RD.txt --PCAfiles ./DATA.RD_PCA \
--normalizeOutput ./DATA.PCA_normalized.txt \
--PCnormalizeMethod PVE_mean --PVE_mean_factor 0.7
Filters and z-score centers (by sample) the PCA-normalized data:
./xhmm --matrix -r ./DATA.PCA_normalized.txt --centerData --centerType sample --zScoreData \
-o ./DATA.PCA_normalized.filtered.sample_zscores.RD.txt \
--outputExcludedTargets ./DATA.PCA_normalized.filtered.sample_zscores.RD.txt.filtered_targets.txt \
--outputExcludedSamples ./DATA.PCA_normalized.filtered.sample_zscores.RD.txt.filtered_samples.txt \
--maxSdTargetRD 30
Filters original read-depth data to be the same as filtered, normalized data:
./xhmm --matrix -r ./DATA.RD.txt \
--excludeTargets ./DATA.filtered_centered.RD.txt.filtered_targets.txt \
--excludeTargets ./DATA.PCA_normalized.filtered.sample_zscores.RD.txt.filtered_targets.txt \
--excludeSamples ./DATA.filtered_centered.RD.txt.filtered_samples.txt \
--excludeSamples ./DATA.PCA_normalized.filtered.sample_zscores.RD.txt.filtered_samples.txt \
-o ./DATA.same_filtered.RD.txt
Discovers CNVs in normalized data:
./xhmm --discover -p ./params.txt \
-r ./DATA.PCA_normalized.filtered.sample_zscores.RD.txt -R ./DATA.same_filtered.RD.txt \
-c ./DATA.xcnv -a ./DATA.aux_xcnv -s ./DATA
NOTE: A description of the .xcnv file format can be found below.
Genotypes discovered CNVs in all samples:
./xhmm --genotype -p ./params.txt \
-r ./DATA.PCA_normalized.filtered.sample_zscores.RD.txt -R ./DATA.same_filtered.RD.txt \
-g ./DATA.xcnv -F ./human_g1k_v37.fasta \
-v ./DATA.vcf
And, the R visualization plots:
Annotate exome targets with their corresponding genes:
pseq . loc-intersect --group refseq --locdb ./RefSeq.locdb --file ./EXOME.interval_list --out ./annotated_targets.refseq
Plot the XHMM pipeline and CNV discovered:
[ First, ensure that the R scripts are installed as described here ]
Rscript example_make_XHMM_plots.R
As an example, the file with parameters: *********************************************************************** Input CNV parameters file: *********************************************************************** 1e-08 6 70 -3 1 0 1 3 1 *********************************************************************** translates into XHMM parameters of: *********************************************************************** Pr(start DEL) = Pr(start DUP) = 1e-08 Mean number of targets in CNV [geometric distribution] = 6 Mean distance between targets within CNV [exponential decay] = 70 KB DEL read depth distribution ~ N(mean=-3, var=1) DIP read depth distribution ~ N(mean=0, var=1) DUP read depth distribution ~ N(mean=3, var=1) ***********************************************************************
SAMPLE CNV INTERVAL KB CHR MID_BP TARGETS NUM_TARG Q_EXACT Q_SOME Q_NON_DIPLOID Q_START Q_STOP MEAN_RD MEAN_ORIG_RD HG00121 DEL 22:18898402-18913235 14.83 22 18905818 104..117 14 9 90 90 8 4 -2.51 37.99 HG00113 DUP 22:17071768-17073440 1.67 22 17072604 4..11 8 25 99 99 53 25 4.00 197.73
| SAMPLE | sample name |
| CNV | type of copy number variation (DEL or DUP) |
| INTERVAL | genomic range of the called CNV |
| KB | length in kilobases of called CNV |
| CHR | chromosome name on which CNV falls |
| MID_BP | the midpoint of the CNV (to have one genomic number for plotting a single point, if desired) |
| TARGETS | the range of the target indices over which the CNV is called (NOTE: considering only the FINAL set of post-filtering targets) |
| NUM_TARG | # of exome targets of the CNV |
| Q_EXACT | Phred-scaled quality of the exact CNV event along the entire interval
- Identical to EQ in .vcf output from genotyping |
| Q_SOME | Phred-scaled quality of some CNV event in the interval
- Identical to SQ in .vcf output from genotyping |
| Q_NON_DIPLOID | Phred-scaled quality of not being diploid, i.e., DEL or DUP event in the interval
- Identical to NDQ in .vcf output from genotyping |
| Q_START | Phred-scaled quality of "left" breakpoint of CNV
- Identical to LQ in .vcf output from genotyping |
| Q_STOP | Phred-scaled quality of "right" breakpoint of CNV
- Identical to RQ in .vcf output from genotyping |
| MEAN_RD | Mean normalized read depth (z-score) over interval
- Identical to RD in .vcf output from genotyping |
| MEAN_ORIG_RD | Mean read depth (# of reads) over interval
- Identical to ORD in .vcf output from genotyping |
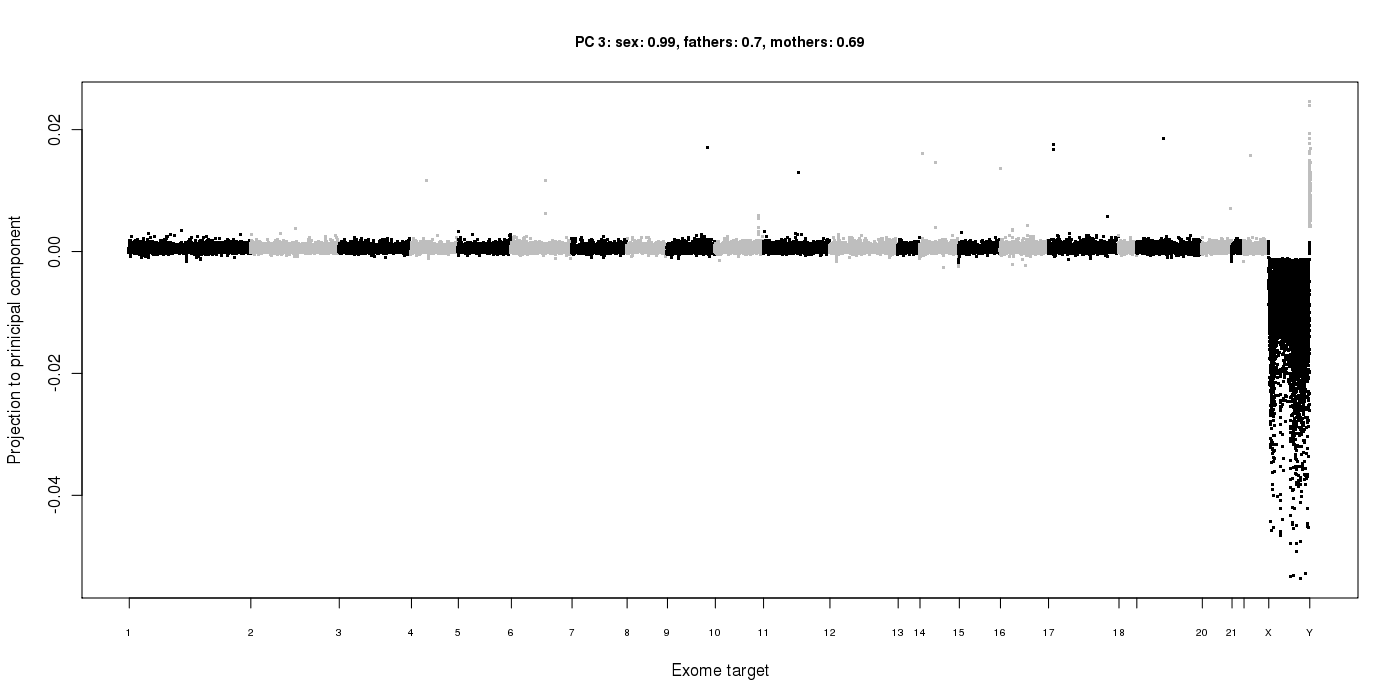
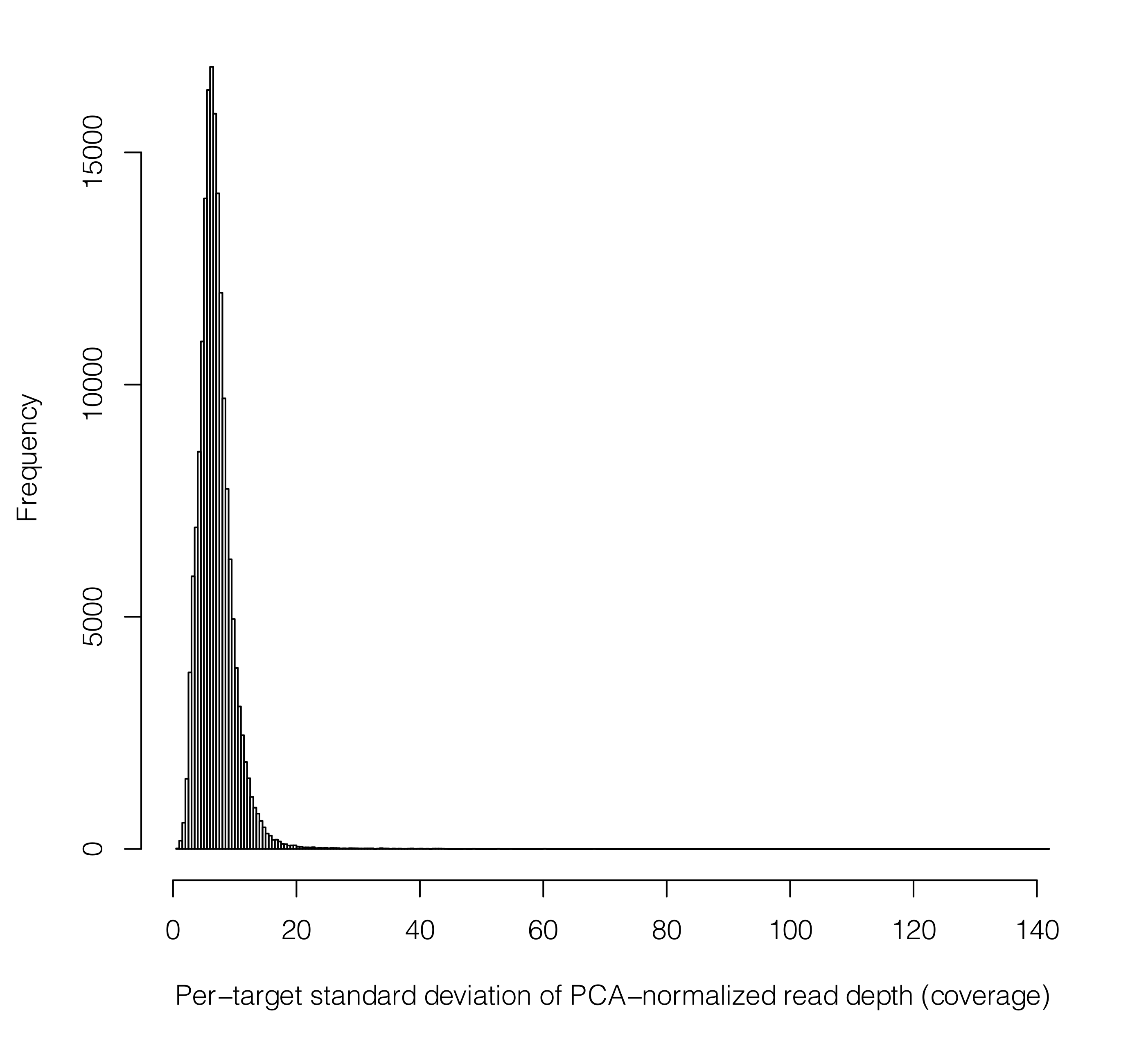
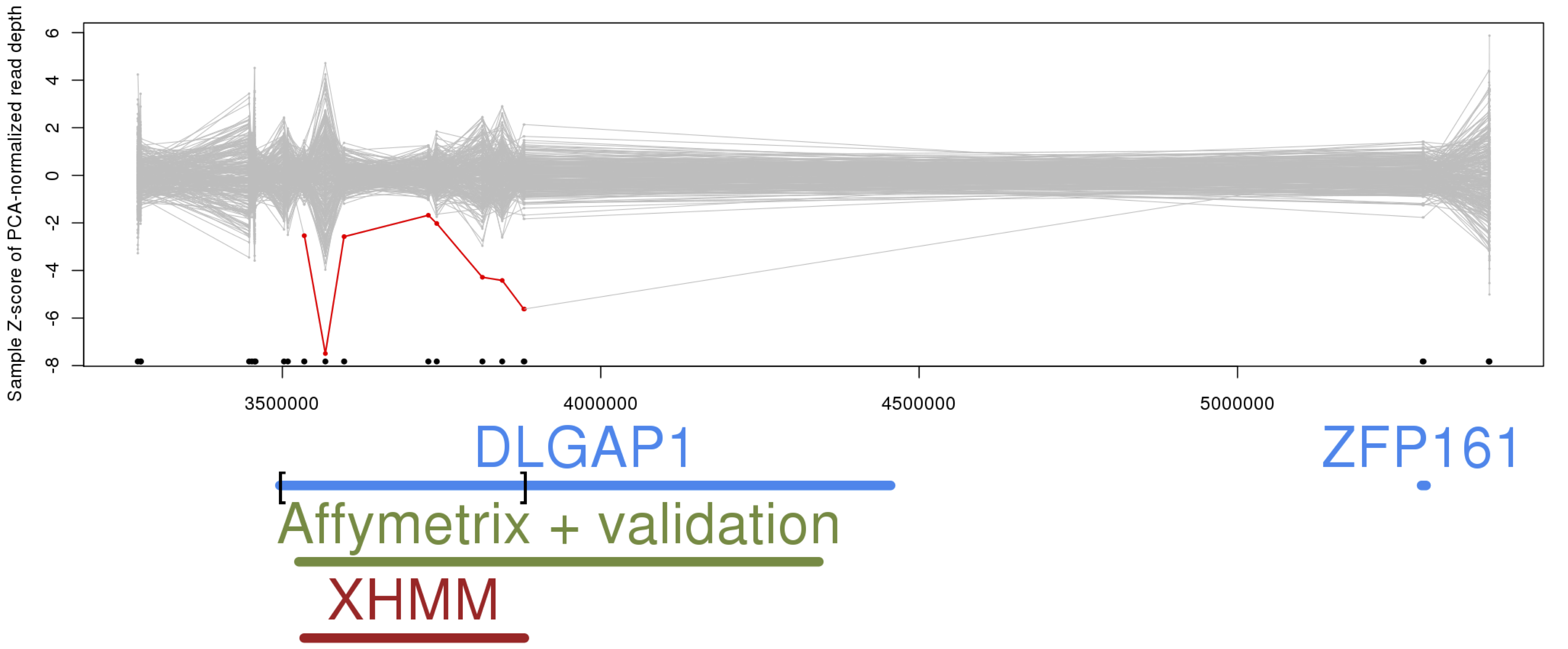
The list below can be obtained by running:
./xhmm -h
-h, --help Print help and exit
--detailed-help Print help, including all details and hidden
options, and exit
--full-help Print help, including hidden options, and exit
-V, --version Print version and exit
*******************************************************************
Options for modes: 'prepareTargets', 'genotype':
-F, --referenceFASTA=STRING Reference FASTA file (MUST have .fai index
file)
*******************************************************************
Options for modes: 'matrix', 'PCA', 'normalize', 'discover', 'genotype':
-r, --readDepths=STRING Matrix of *input* read-depths, where rows
(samples) and columns (targets) are labeled
(default=`-')
*******************************************************************
Mode: prepareTargets
Sort all target intervals, merge overlapping ones, and print the resulting
interval list
-S, --prepareTargets
--targets=STRING Input targets lists
--mergedTargets=STRING Output targets list (default=`-')
Mode: mergeGATKdepths
Merge the output from GATK into a single read depth matrix of samples (rows)
by targets (columns)
-A, --mergeGATKdepths
--GATKdepths=STRING GATK sample_interval_summary output file(s) to
be merged [must have *IDENTICAL* target
lists]
--GATKdepthsList=STRING A file containing a list of GATK
sample_interval_summary output files to be
merged [must have *IDENTICAL* target lists]
--sampleIDmap=STRING File containing mappings of sample names to new
sample names (in columns designated by
fromID, toID)
--fromID=INT Column number of OLD sample IDs to map
(default=`1')
--toID=INT Column number of NEW sample IDs to map
(default=`2')
Mode: matrix
Process (filter, center, etc.) a read depth matrix and output the resulting
matrix. Note that first all excluded samples and targets are removed. And,
sample statistics used for filtering are calculated only *after* filtering
out relevant targets.
-M, --matrix
--excludeTargets=STRING File(s) of targets to exclude
--excludeChromosomeTargets=STRING
Target chromosome(s) to exclude
--excludeSamples=STRING File(s) of samples to exclude
--minTargetSize=INT Minimum size of target (in bp) to process
(default=`0')
--maxTargetSize=INT Maximum size of target (in bp) to process
--minMeanTargetRD=DOUBLE Minimum per-target mean RD to require for
target to be processed
--maxMeanTargetRD=DOUBLE Maximum per-target mean RD to require for
target to be processed
--minSdTargetRD=DOUBLE Minimum per-target standard deviation of RD to
require for target to be processed
(default=`0')
--maxSdTargetRD=DOUBLE Maximum per-target standard deviation of RD to
require for target to be processed
--minMeanSampleRD=DOUBLE Minimum per-sample mean RD to require for
sample to be processed
--maxMeanSampleRD=DOUBLE Maximum per-sample mean RD to require for
sample to be processed
--minSdSampleRD=DOUBLE Minimum per-sample standard deviation of RD to
require for sample to be processed
(default=`0')
--maxSdSampleRD=DOUBLE Maximum per-sample standard deviation of RD to
require for sample to be processed
--centerData Output sample- or target- centered read-depth
matrix (as per --centerType) (default=off)
--centerType=ENUM If --centerData given, then center the data
around this dimension (possible
values="target", "sample")
--zScoreData If --centerData given, then additionally
normalize by standard deviation (outputting
z-scores) (default=off)
--outputExcludedTargets=STRING
File in which to output targets excluded by
some criterion
--outputExcludedSamples=STRING
File in which to output samples excluded by
some criterion
Options for modes: 'mergeGATKdepths', 'matrix':
-o, --outputMatrix=STRING Read-depth matrix output file (default=`-')
*******************************************************************
Mode: PCA
Run PCA to create normalization factors for read depth matrix
-P, --PCA Matrix is read from --readDepths argument;
normalization factors sent to --PCAfiles
argument
Mode: normalize
Apply PCA factors in order to normalize read depth matrix
-N, --normalize Matrix is read from --readDepths argument;
normalization factors read from --PCAfiles
argument
-n, --normalizeOutput=STRING Normalized read-depth matrix output file
(default=`-')
--PCnormalizeMethod=ENUM Method to choose which prinicipal components
are removed for data normalization (possible
values="numPCtoRemove", "PVE_mean",
"PVE_contrib" default=`PVE_mean')
--numPCtoRemove=INT Number of highest principal components to
filter out (default=`20')
--PVE_mean_factor=DOUBLE Remove all principal components that
individually explain more variance than this
factor times the average (in the original
PCA-ed data) (default=`0.7')
--PVE_contrib=DOUBLE Remove the smallest number of principal
components that explain this percent of the
variance (in the original PCA-ed data)
(default=`50')
Options for modes: 'PCA', 'normalize':
--PCAfiles=STRING Base file name for 'PCA' *output*, and
'normalize' *input*
*******************************************************************
Mode: discover
Discover CNVs from normalized read depth matrix
-D, --discover Matrix is read from --readDepths argument
-c, --xcnv=STRING CNV output file (default=`-')
-t, --discoverSomeQualThresh=DOUBLE
Quality threshold for discovering a CNV in a
sample (default=`30')
-s, --posteriorFiles=STRING Base file name for posterior probabilities
output files; if not given, and --xcnv is not
'-', this will default to --xcnv argument
Mode: genotype
Genotype list of CNVs from normalized read depth matrix
-G, --genotype Matrix is read from --readDepths argument
-g, --gxcnv=STRING xhmm CNV input file to genotype in 'readDepths'
sample
-v, --vcf=STRING Genotyped CNV output VCF file (default=`-')
--subsegments In addition to genotyping the intervals
specified in gxcnv, genotype all sub-segments
of these intervals (with
maxTargetsInSubsegment or fewer targets)
(default=off)
--maxTargetsInSubsegment=INT
When genotyping sub-segments of input
intervals, only consider sub-segments
consisting of this number of targets or fewer
(default=`30')
-T, --genotypeQualThresholdWhenNoExact=DOUBLE
Quality threshold for calling a genotype, used
*ONLY* when 'gxcnv' does not contain the
'Q_EXACT' field for the interval being
genotyped (default=`20')
Options for modes: 'discover', 'genotype', 'transition', 'printHMM':
-p, --paramFile=STRING (Initial) model parameters file
Options for modes: 'discover', 'genotype':
-m, --maxNormalizedReadDepthVal=DOUBLE
Value at which to cap the absolute value of
*normalized* input read depth values
('readDepths') (default=`10')
-q, --maxQualScore=DOUBLE Value at which to cap the calculated quality
scores (default=`99')
-e, --scorePrecision=INT Decimal precision of quality scores
(default=`0')
-a, --aux_xcnv=STRING Auxiliary CNV output file (may be VERY LARGE in
'genotype' mode)
-u, --auxUpstreamPrintTargs=INT
Number of targets to print upstream of CNV in
'auxOutput' file (default=`2')
-w, --auxDownstreamPrintTargs=INT
Number of targets to print downstream of CNV in
'auxOutput' file (default=`2')
-R, --origReadDepths=STRING Matrix of unnormalized read-depths to use for
CNV annotation, where rows (samples) and
columns (targets) are labeled
*******************************************************************
Mode: printHMM
Print HMM model parameters and exit
--printHMM
Mode: transition
Print HMM transition matrix for user-requested genomic distances
--transition